Cryopreservation in Cell Therapy Workflows: Overcoming the Limitations of DMSO-Based Formulations
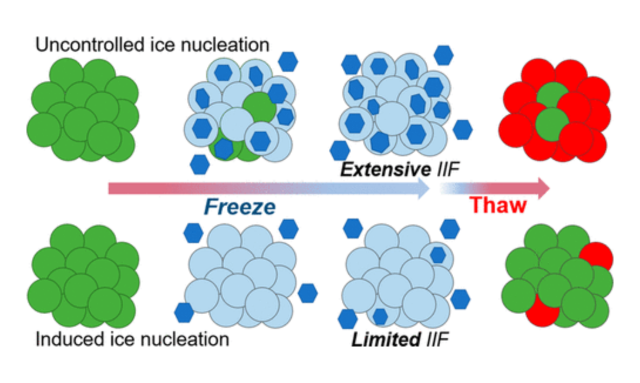
Cryopreservation is a crucial step in cell therapy workflows, ensuring the long-term viability and availability of therapeutic cells. However, the use of traditional dimethyl sulfoxide (DMSO) cryoprotectants presents challenges due to associated toxicities. This article highlights the importance of cryopreservation in cell therapy, examines the potential harms of DMSO, and discusses relevant studies that shed...
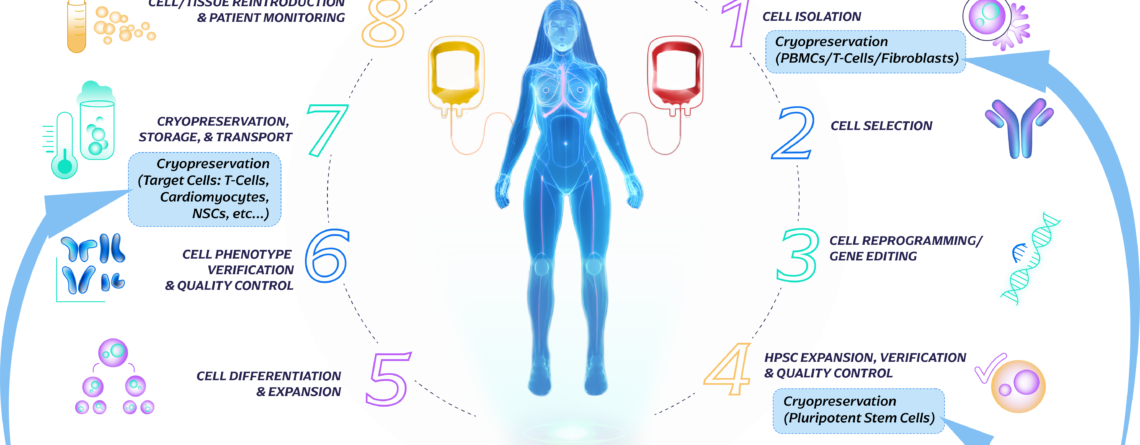
Introduction to the Cell Therapy Workflow
Allogeneic Workflow: In the allogeneic approach, immune cells are sourced from healthy donors or established cell banks rather than the patient themselves. These donor cells are carefully selected and expanded in large quantities under controlled laboratory conditions. The expanded cells are then modified or activated to enhance their therapeutic properties, such as targeting specific disease markers...
What is CGMP?
Knowing the current good manufacturing practices (cGMP) in the industry of Pharmaceutical. Initially, it looks as if trying to have a handful of water. It is a complicated concept that is difficult to hold together. Currently, the FDA offers 34 distinct final guidance documents for the cGMP in the pharmaceutical industry, demanding process validations,...