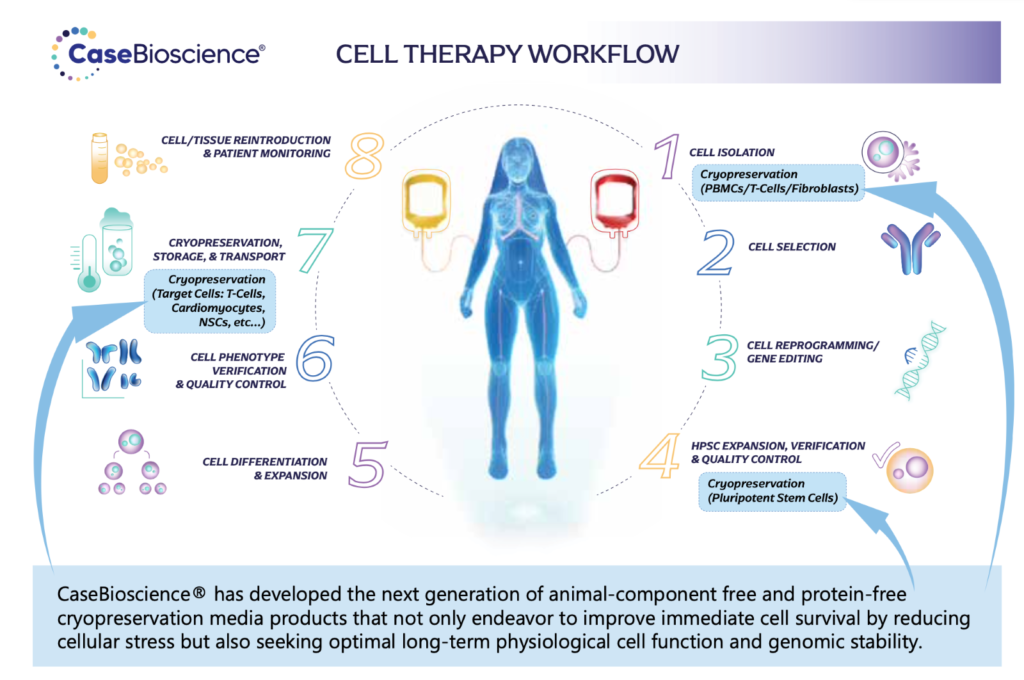
Cryopreservation in Cell Therapy Workflows: Overcoming the Limitations of DMSO-Based Formulations
Cryopreservation is a crucial step in cell therapy workflows, ensuring the long-term viability and availability of therapeutic cells. However, the use of traditional dimethyl sulfoxide (DMSO) cryoprotectants presents challenges due to associated toxicities. This article highlights the importance of cryopreservation in cell therapy, examines the potential harms of DMSO, and discusses relevant studies that shed light on its cytotoxic effects and limitations.
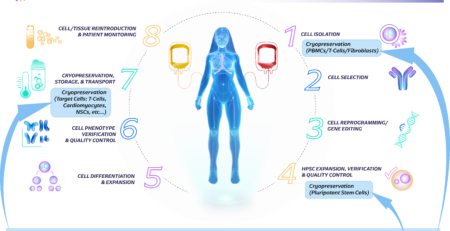
Cell therapy has emerged as a promising approach for treating various diseases. Cryopreservation is a key element in cell therapy workflows, allowing for the storage, transportation, and accessibility of therapeutic cells. However, the conventional use of DMSO as a cryoprotectant raises concerns due to its potential adverse effects on cell viability and functionality.
Limitations and Harmful Effects of DMSO:
DMSO, although widely used as a cryoprotectant, has been associated with cytotoxic effects that can impair the integrity and function of cells. Studies have shown that DMSO-induced cytotoxicity can lead to cellular membrane damage, alterations in intracellular signaling pathways, and compromised cell survivali. Additionally, DMSO may induce oxidative stress, disrupt cell differentiation, and trigger immunological responses, which may hinder the therapeutic potential of cryopreserved cells.
Studies Demonstrating DMSO-Induced Toxicity:
Several scientific investigations have shed light on the harmful effects of DMSO in cell therapies. Comprehensive reviews of the published literature identified several hundred adverse reactions (e.g. cardiac arrhythmias, neurological symptoms and respiratory arrest) associated with the transplantation of stem cells cryopreserved with DMSO ii iii Furthermore, according to the European society of Blood and Marrow Transplantation (EBMT) analysis, around 30–60% of DMSO-containing transplants are associated with at least one side effect or complicationiv v. DMSO even at low concentrations has shown toxic properties in the blood cells, inducing RBCs hemolysis, reduction in platelet activities, and reduction of platelet aggregation levelsvi. In addition, DMSO has been shown to damage mitochondrial integrity and membrane potential in cultured astrocytesvii. Direct cellular toxicity has also been shown in other CNS cells such as neuronsviii, as well as oligodendrocytes where a concentration of 1% DMSO robustly suppressed oligodendrogenesis and drove the fate of differentiating neural stem/progenitor cells (NSPCs) toward astrogenesisix. Review of literature sheds further light into the cellular toxicity of DMSO across several different cell types.
Advancements in Non-DMSO Cryopreservation Solutions:
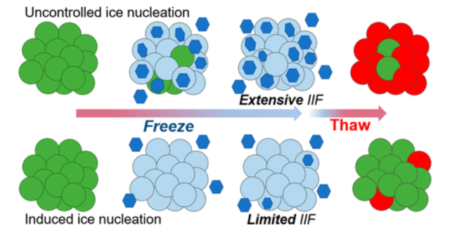
Recognizing the limitations of DMSO-based formulations, researchers and industry pioneers are actively exploring non-DMSO alternatives for cryopreservation. Novel cryoprotective agents, such as natural polymers, synthetic compounds, and ice-recrystallization inhibitors, are being investigated for their efficacy in maintaining cell viability and functionality post-thaw. Furthermore, advancements in freezing and thawing protocols, alongside improved storage conditions, contribute to the development of safer and more effective cryopreservation strategies.
Conclusion:
Cryopreservation is a critical component of successful cell therapy workflows, ensuring the availability and quality of therapeutic cells. However, the potential harms associated with traditional DMSO cryoprotectants necessitate the development of alternative solutions. Numerous studies have demonstrated the cytotoxic effects of DMSO on cellular viability and function. By embracing non-DMSO cryopreservation approaches, we can improve the safety and efficacy of cell therapies, ultimately benefiting patients and advancing the field of regenerative medicine.
The utilization of DMSO in cryopreservation poses significant challenges due to its potential harmful effects on cells. Studies have highlighted the cytotoxicity of DMSO and its impact on cell viability and functionality. By focusing on the development of non-DMSO cryopreservation solutions and further research in this area, we can mitigate the limitations associated with DMSO and enhance the success of cell therapies in clinical applications.
In conclusion, removal of DMSO from cryopreserved cell therapy procedures such as human stem cell preservation is an unmet need. Replacement with a non-toxic cryoprotectant that is not metabolized by humans would remove the need for complicated post-thaw handling procedures and minimize costs.
i Dimethyl sulfoxide: a central player since the dawn of cryobiology, is efficacy balanced by toxicity?Maooz Awan, Iryna Buriak, Roland Fleck, Barry Fuller, Anatoliy Goltsev, Julie Kerby, Mark Lowdell, Pavel Mericka, Alexander Petrenko, Yuri Petrenko, Olena Rogulska, Alexandra Stolzing, and Glyn N Stace Regenerative Medicine 2020 15:3, 1463-1491
ii Shu Z, Heimfeld S, Gao D. Hematopoietic SCT with cryopreserved grafts: adverse reactions after transplantation and cryoprotectant removal before infusion. Bone Marrow Transplant. 2014 Apr;49(4):469-76. doi: 10.1038/bmt.2013.152. Epub 2013 Sep 30. PMID: 24076548; PMCID: PMC4420483.
iii Zeng X, Zhao C, Wang H, et al. Dimethyl Sulfoxide Decrease Type–I and –III Collagen Synthesis in Human Hepatic Stellate Cells and Human Foreskin Fibroblasts. Advanced Science Letters. 2010;3:496–499
iv Nusbaumer D , Cunha LM da , Wedekind C . Comparing methanol-glucose and dimethyl-sulfoxide based extender for milt cryopreservation of brown trout (Salmo trutta). BioRxiv.doi: https://doi.org/10.1101/289736 289736 (2018)
v Aramli MS , Golshahi K , Nazari RM , Aramli S , Banan A . Effectiveness of glucose–methanol extender for cryopreservation of Huso huso spermatozoa. Anim. Reprod. Sci. 162, 37–42 (2015).
vi Toxic effects of dimethyl sulfoxide on red blood cells, platelets, and vascular endothelial cells in vitro. Xiaoyang Yi, Minxia Liu, Qun Luo, Hailong Zhuo, Hui Cao, Jiexi Wang, Ying Han
First published: 08 January 2017
vii Yuan C, Gao J, Guo J, Bai L, Marshall C, Cai Z, Wang L, Xiao M. Dimethyl sulfoxide damages mitochondrial integrity and membrane potential in cultured astrocytes. PLoS One. 2014 Sep 19;9(9):e107447. doi: 10.1371/journal.pone.0107447. PMID: 25238609; PMCID: PMC4169574.
viii Hanslick JL, Lau K, Noguchi KK, Olney JW, Zorumski CF, Mennerick S, Farber NB. Dimethyl sulfoxide (DMSO) produces widespread apoptosis in the developing central nervous system. Neurobiol Dis. 2009 Apr;34(1):1-10. doi: 10.1016/j.nbd.2008.11.006. Epub 2008 Dec 3. PMID: 19100327; PMCID: PMC2682536
ix Dimethylsulfoxide Inhibits Oligodendrocyte Fate Choice of Adult Neural Stem and Progenitor Cell Front. Neurosci., 26 November 2019
Sec. Neurogenesis Volume 13 – 2019 | https://doi.org/10.3389/fnins.2019.01242







Leave a Reply