Introduction to the Cell Therapy Workflow
Allogeneic Workflow:
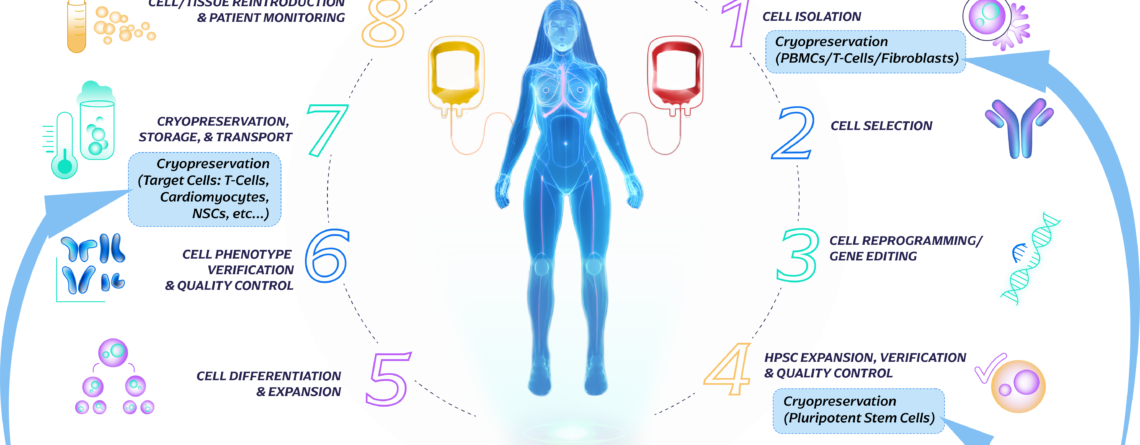
In the allogeneic approach, immune cells are sourced from healthy donors or established cell banks rather than the patient themselves. These donor cells are carefully selected and expanded in large quantities under controlled laboratory conditions. The expanded cells are then modified or activated to enhance their therapeutic properties, such as targeting specific disease markers or boosting immune responses. Once the cells are ready, they are cryopreserved for later use in multiple patients. The advantage of allogeneic therapy is that it enables the production of off-the-shelf cell products that can be readily available for a wide range of patients, eliminating the need for patient-specific manufacturing.
Autologous Workflow:
In the autologous approach, immune cells are derived from the patient receiving the therapy. A sample of the patient’s own immune cells, such as T cells or natural killer (NK) cells, is collected through a process called leukapheresis. These cells are then sent to a specialized laboratory where they are isolated, expanded, and genetically modified or activated to enhance their therapeutic potential. Once the cells have been manipulated, they are administered back to the patient, usually following a conditioning regimen to prepare the body for their infusion. The advantage of the autologous approach is that it offers a personalized treatment strategy, utilizing the patient’s own cells to minimize the risk of immune rejection and potentially improve efficacy.
It’s important to note that both allogeneic and autologous workflows have their advantages and considerations. Allogeneic therapies provide a readily available cell product with potential cost and scalability advantages but may require careful matching and immunosuppressive strategies to avoid graft-versus-host disease (GVHD). Autologous therapies offer personalized treatment options but can involve longer manufacturing times and higher costs due to the need for individual patient processing.
The choice between allogeneic and autologous approaches depends on factors such as the specific therapeutic goals, the nature of the disease being targeted, patient eligibility, and regulatory considerations. Researchers and clinicians continue to explore and refine these workflows to harness the full potential of immune cell therapies in addressing a wide range of diseases and conditions.
Cryopreservation steps:
Throughout the immune cell therapy workflow, cryopreservation steps are strategically implemented to ensure the availability of viable and functional cells at the desired time. This allows for flexibility in treatment schedules, batch manufacturing, and long-term storage, while preserving the integrity and potency of the therapeutic cells. The optimized cryopreservation and storage of immune cells are vital in facilitating successful immune cell therapy treatments and improving patient outcomes. Throughout the cycle of immune cell therapy, there are several points where cryopreservation and storage steps are involved. Some of these points have been highlighted in the illustration below.
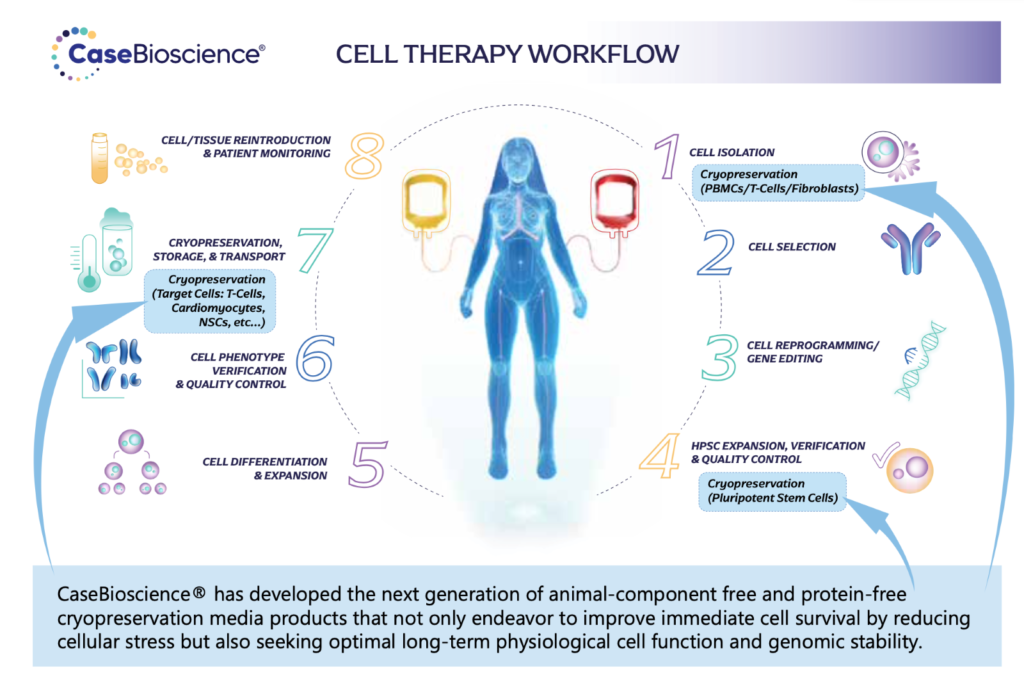
Immune cell therapies hold immense promise in treating various diseases and conditions. Here are some examples of diseases where these treatments have shown success:
Cancer: Immune cell therapies, such as chimeric antigen receptor (CAR) T-cell therapy, have demonstrated remarkable efficacy in certain types of blood cancers, including leukemia and lymphoma. These therapies involve engineering patient or donor immune cells to recognize and target cancer cells, leading to significant remission rates and improved survival outcomes. Since 2017 there have been 6 FDA approved CAR-T Therapies which have all produced meaningful response rates across various indications. i
Autoimmune Disorders: Immune cell therapies have shown potential in addressing autoimmune diseases, where the immune system mistakenly attacks healthy tissues. For example, therapies using regulatory T cells (Tregs) aim to modulate the immune response and restore immune tolerance, offering potential treatment options for diseases like rheumatoid arthritis, multiple sclerosis, and type 1 diabetes.
Infectious Diseases: Immune cell therapies, particularly adoptive T-cell transfer, have been explored for combating viral infections, such as human immunodeficiency virus (HIV) and viral hepatitis. These therapies involve infusing patient-derived or engineered T cells with enhanced antiviral properties to target and eliminate infected cells.
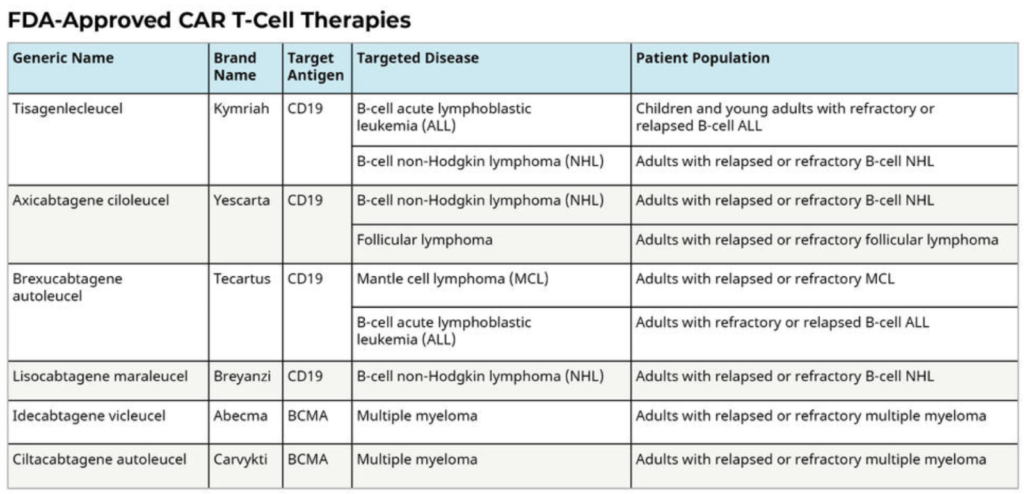
CAR T Cells: Engineering Patients’ Immune Cells to Treat Their Cancers was originally published by the National Cancer Institute. https://www.cancer.gov/about-cancer/treatment/research/car-t-cells
Current Limitations:
While immune cell therapies have shown remarkable success in some cases, there are also limitations and challenges to consider:
Limited Applicability: Currently, immune cell therapies are primarily focused on hematologic malignancies and select autoimmune disorders. Expanding their application to solid tumors and other diseases remains an ongoing challenge due to factors like tumor heterogeneity, immunosuppressive microenvironments, and the need for more precise targeting strategies.
Manufacturing Complexities: The manufacturing process of immune cell therapies is intricate and time-consuming, involving cell isolation, genetic modification, expansion, and quality control. Streamlining and optimizing manufacturing processes are crucial for broader patient access and scalability.
Safety Concerns: Immune cell therapies can elicit severe adverse effects, including cytokine release syndrome and neurotoxicity. Developing strategies to mitigate these risks and enhance safety profiles is essential for broader adoption and improved patient outcomes.
The Future:
The future of immune cell therapy workflows holds great potential for advancements and improvements. Here are key areas of focus:
Precision and Personalization: Advancing therapies that target specific disease markers or antigens with high precision, allowing for personalized treatment approaches tailored to each patient’s unique profile.
Combination Therapies: Exploring synergistic combinations of immune cell therapies with other treatment modalities, such as checkpoint inhibitors, targeted therapies, or radiation, to enhance efficacy and overcome resistance mechanisms.
Off-the-Shelf Products: Advancing allogeneic cell therapies that can be readily available and universally applicable, reducing the time and costs associated with patient-specific manufacturing.
Overcoming Immune Evasion: Developing strategies to overcome immune evasion mechanisms employed by tumors or diseases, enabling immune cells to effectively recognize and eliminate targeted cells.
Enhanced Safety Profiles: Designing approaches to minimize severe adverse effects and improving the overall safety profile of immune cell therapies.
Conclusion:
immune cell therapies have shown great promise in treating various diseases, particularly in cancer and autoimmune disorders. While there are limitations and challenges to address, ongoing research and advancements in the field are paving the way for improved efficacy, broader applicability, and enhanced patient outcomes. Continued innovation and collaboration among researchers, clinicians, and industry stakeholders are crucial for the future success of immune cell therapy workflows.
i CAR T Cells: Engineering Patients’ Immune Cells to Treat Their Cancers was originally published by the National Cancer Institute. https://www.cancer.gov/about-cancer/treatment/research/car-t-cells








Leave a Reply