Stem Cell Therapy Workflow
admin2024-01-18T22:25:16+00:00Stem Cell Therapy Workflow
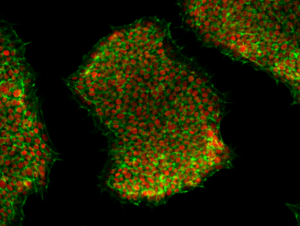
The transition from stem cell research to clinical applications such as regenerative stem cell therapies require high quality cGMP bio-solutions.
In particular, the promise of human pluripotent stem cell (hPSC)-based cell therapies is being realized with the growing number of clinical trials (Blau and Daley, 2019; Ilic and Ogilvie, 2022).
There are a few key steps in cell therapy workflows that require cryopreservation, which allows for banking and long-term storage of stem cells and stem-cell derivatives that can be used at multiple time-points and potentially with multiple patients for allogenic therapies. Currently, most laboratories use DMSO based cryopreservation solutions that contain animal serum, human serum or serum derived proteins which can be limiting and undefined.
CaseBioscience has developed the next generation of animal-component free and protein-free cryopreservation media products that not only endeavor to improve immediate cell survival by reducing cellular stress but also seeking optimal long-term physiological cell function and genomic stability.

CaseCryo® NON-DMSO “The scientific team at CaseBioscience with its leading cryobiologists, cell biologists, and development biologists aims to bring market[1]disruptive, innovative non-DMSO formulations and improved methods of cryopreservation that offer safer and accessible alternatives in cell and gene therapies” says Chief Scientific Officer, Kevin Flynn, PhD.
Through the Case media platform, and a range of media optimization and manufacturing services, we offer advanced cell culture and cryopreservation media solutions for stem and primary cells to support basic, translational, and clinical research, as well as commercial applications.
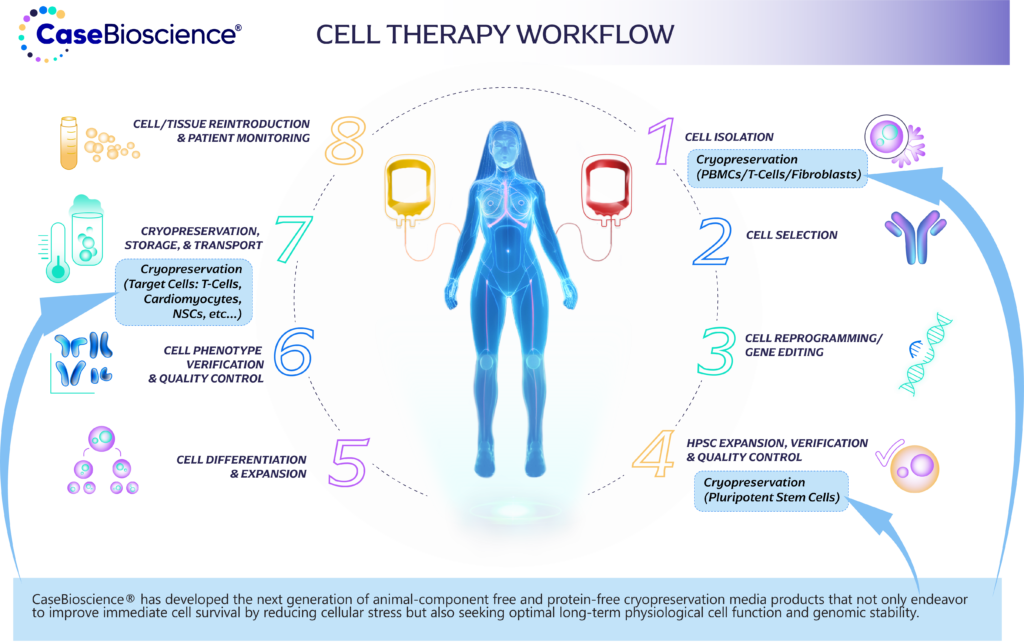
CaseBioscience is dedicated to developing a comprehensive range of products tailored to various stage of the cell therapy workflow. Our innovative solutions address the unique challenges at each step, from cell culture and expansion to cryopreservation and quality control. Experience a seamless and efficient cell therapy journey with our cutting-edge products designed to support researchers and clinicians at every crucial stage.
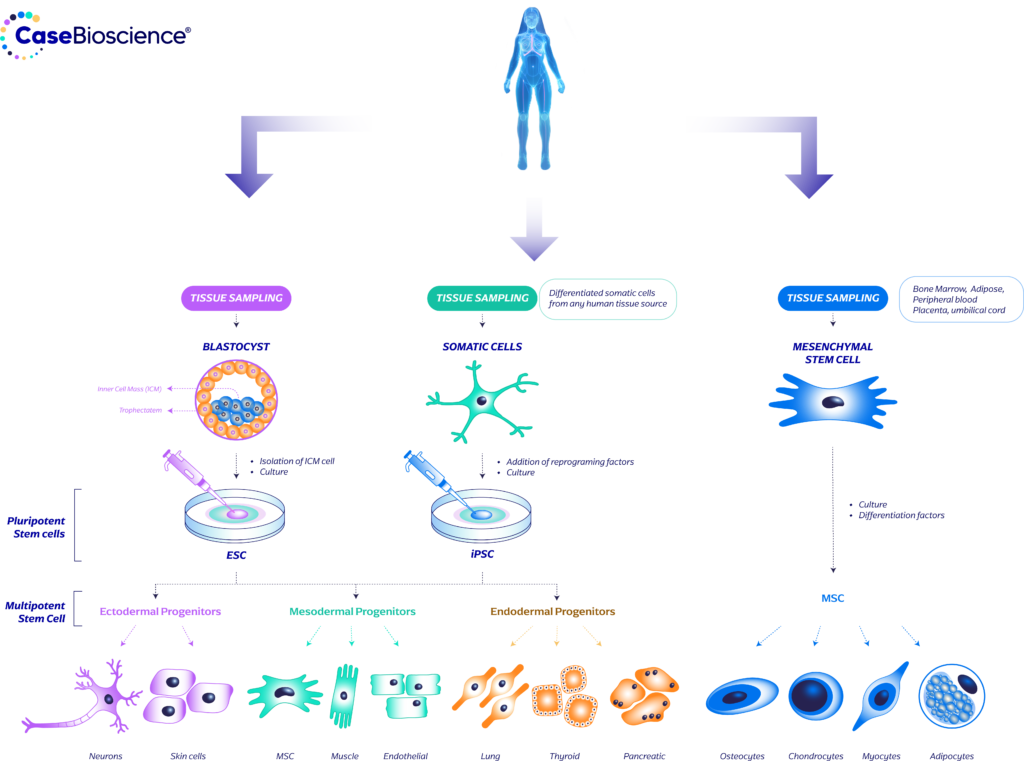
While MSCs, iPSCs, and ESCs have distinct differences in collection methods and ethical considerations, all three types of stem cells hold significant therapeutic potential. The choice of stem cell type depends on the specific application, disease, and patient considerations. Ongoing research and technological advancements continue to enhance our understanding of these stem cell types and their potential for clinical applications.