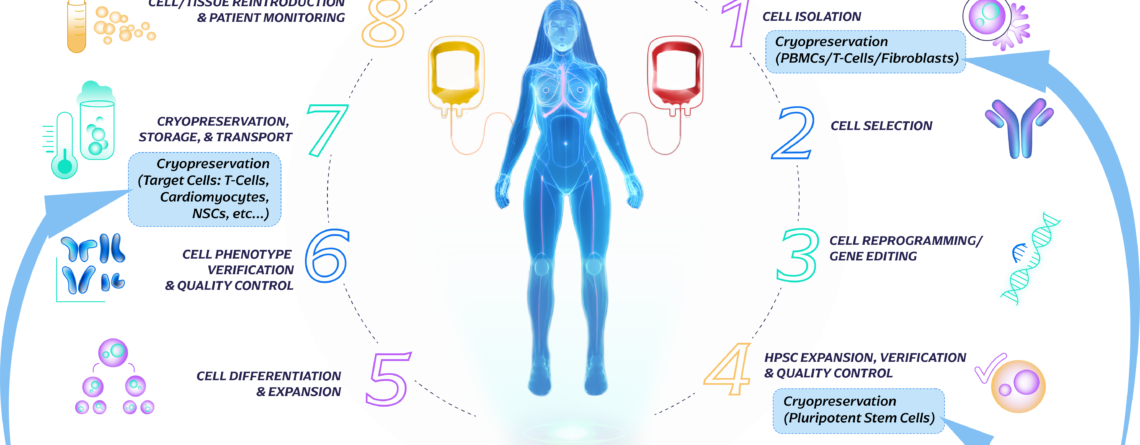
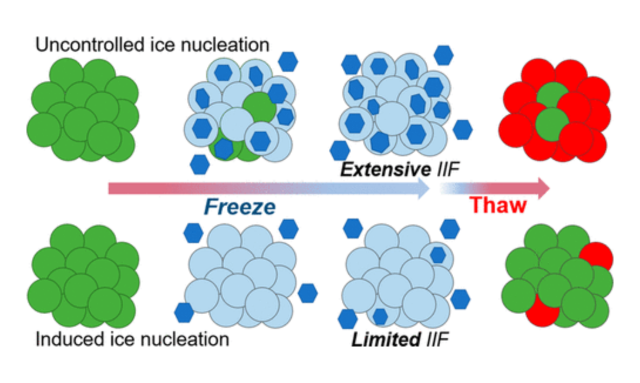
Introduction to the Cell Therapy Workflow
Allogeneic Workflow: In the allogeneic approach, immune cells are sourced from healthy donors or established cell banks rather than the patient themselves. These donor cells are carefully selected and expanded in large quantities under controlled laboratory conditions. The expanded cells are then modified or activated to enhance their therapeutic properties, such as targeting specific disease markers...